Ruxolitinib improves the inflammatory microenvironment,restores glutamate homeostasis,and promotes functional recovery after spinal cord injury
Jiang Cao ,Xiao Yu ,Jingcheng Liu ,Jiaju Fu,Binyu Wang,Chaoqin Wu,Sheng Zhang,Hongtao Chen,Zi Wang,Yinyang Xu,Tao Sui,*,Jie Chang,*,Xiaojian Cao,*
Abstract The inflammatory microenvironment and neurotoxicity can hinder neuronal regeneration and functional recovery after spinal cord injury.Ruxolitinib,a JAK-STAT inhibitor,exhibits effectiveness in autoimmune diseases,arthritis,and managing inflammatory cytokine storms.Although studies have shown the neuroprotective potential of ruxolitinib in neurological trauma,the exact mechanism by which it enhances functional recovery after spinal cord injury,particularly its effect on astrocytes,remains unclear.To address this gap,we established a mouse model of T10 spinal cord contusion and found that ruxolitinib effectively improved hindlimb motor function and reduced the area of spinal cord injury.Transcriptome sequencing analysis showed that ruxolitinib alleviated inflammation and immune response after spinal cord injury,restored EAAT2 expression,reduced glutamate levels,and alleviated excitatory toxicity.Furthermore,ruxolitinib inhibited the phosphorylation of JAK2 and STΑT3 in the injured spinal cord and decreased the phosphorylation level of nuclear factor kappa-B and the expression of inflammatory factors interleukin-1β,interleukin-6,and tumor necrosis factor-α.Αdditionally,in glutamate-induced excitotoxicity astrocytes,ruxolitinib restored EΑΑT2 expression and increased glutamate uptake by inhibiting the activation of STΑT3,thereby reducing glutamate-induced neurotoxicity,calcium influx,oxidative stress,and cell apoptosis,and increasing the complexity of dendritic branching.Collectively,these results indicate that ruxolitinib restores glutamate homeostasis by rescuing the expression of EAAT2 in astrocytes,reduces neurotoxicity,and effectively alleviates inflammatory and immune responses after spinal cord injury,thereby promoting functional recovery after spinal cord injury.
Key Words: astrocytes;EAAT2;excitotoxicity;glutamate homeostasis;JAK-STAT pathway;locomotor function;neurotoxicity;ruxolitinib;spinal cord injury;transcriptome analysis
Introduction
Spinal cord injury (SCI) typically leads to the loss of limb function below the level of the injury (McDonald and Sadowsky,2002).The initial trauma causes immediate bleeding and cell death,which limits the time for potential therapeutic interventions.After the initial injury,a cascade of secondary damage ensues,characterized by pervasive and sustained inflammation and progressive tissue loss (Ahuja et al.,2017;Li et al.,2020).Secondary damage arises from various factors,including inflammation,cellular injuries prompted by reactive oxygen species (ROS) and reactive nitrogen species,glutamate-induced excitotoxicity,and calcium influx (Jia et al.,2012;Zhou et al.,2020;Hellenbrand et al.,2021;Bretheau et al.,2022;Zhao et al.,2022;Chen et al.,2023;Liu et al.,2023;Wang et al.,2023).The extent of damage during secondary injury may be higher than that incurred during the initial trauma.Consequently,strategies targeting the mitigation of secondary injury progression hold significant promise as a clinically feasible approach to reduce tissue damage and mitigate functional deficits after SCI(Hausmann,2003;Hu et al.,2023).In animals,SCI is primarily treated with drugs,surgery,and cell-based therapies.However,there is no effective treatment for SCI in humans.Several treatment methods are in the early stages of development with unconfirmed clinical effects (Serafin et al.,2022,2023).Clinical trials focused on improving functional recovery after SCI are limited and often yield unsatisfactory findings (Garcia et al.,2023;Li et al.,2023a;Patil et al.,2023;Quddusi et al.,2023;Watson et al.,2023).Therefore,developing effective treatment strategies for SCI remains crucial.
Astrocytes,the predominant cellular component of the central nervous system,play a supportive role for neurons and have a pivotal function in regulating glutamate homeostasis within the central nervous system (Lattke et al.,2021;Burda et al.,2022;Valles et al.,2023).Following SCI,astrocytes not only contribute to the structural and functional maintenance of neurons but also become key players in the inflammatory response and glutamate homeostasis.Αctivated microglia release interleukin (IL)-1α,tumor necrosis factor-α (TNF-α),and complement 1q (C1q) after SCI,triggering astrocyte activation and swelling,and transforming them into neurotoxic,A1-like,reactive astrocytes (Liddelow et al.,2017;O’Shea et al.,2017;Lukacova et al.,2021).Along with other immune cells,the reactive astrocytes release inflammatory mediators such as cytokines and chemokines,resulting in neuronal injury and secondary injury diffusion (Kwiecien et al.,2020;Zhang et al.,2023;Hellenbrand et al.,2024).
Excitatory amino-acid transporter 2 (EAAT2),the primary glutamate transporter in the central nervous system,is predominantly expressed in astrocytes (Rimmele and Rosenberg,2016;Pajarillo et al.,2019).EAAT2 expression in astrocytes substantially decreases after SCI,and the downregulation persists for up to 6 weeks (Lepore et al.,2011a,b).This reduction leads to the release of glutamate from damaged neurons and astrocytes in the spinal cord,resulting in calcium influx in neurons,followed by oxidative stress,excitatory toxicity,and aggravated neuronal damage (Αlijanpour et al.,2023;Huang et al.,2023).Consequently,sustained downregulation of EAAT2 significantly worsens the outcome of SCI (Lepore et al.,2011a).The crucial role of EAAT2 in neuroprotection has been confirmed in other neurological conditions.For example,EΑΑT2 knockout led to lifethreatening spontaneous seizures and substantial neuronal loss in mice,whereas functional EAAT2 prevented post-traumatic seizures in rat models of brain trauma (Dorsett et al.,2017;Liu et al.,2020).
Signal transducer and activator of transcription 3 (STΑT3),a crucial signal transduction molecule,is an important regulator in SCI.Αctivation of STΑT3 in astrocytes correlates with inflammation,astrocyte activation,and neuronal survival (Herrmann et al.,2008;Wang et al.,2021;Zhou et al.,2023).Ruxolitinib (RUX)is a Janus kinase (JAK) inhibitor that is used in the treatment of myelofibrosis and polycythemia vera and other immune diseases (Ju et al.,2016;Lee et al.,2021).RUX has demonstrated remarkable neuroprotective activity in mouse models of human immunodeficiency virus encephalitis.RUX effectively suppresses astrocyte proliferation by inhibiting JΑK2-STΑT3 signaling without inducing notable toxicity (Haile et al.,2016).Although previous reports indicated RUX’s neuroprotective potential in nervous system trauma (Chen et al.,2021;Qian et al.,2022),its precise mechanism for enhancing functional recovery following SCI,particularly in terms of its effect on astrocytes,has not been investigated.This study aimed to examine the potential of RUX to alleviate astrocyte-mediated inflammatory responses and restore glutamate homeostasis in SCI.
Methods
Animals
We procured specific-pathogen-free robust female adult C57BL/6J mice,8 weeks of age and weighing 18–20 g,from Animal Core Facility of Nanjing Medical University (license No.SCXK (Su) 2021-0001).Male mice were not used because of their susceptibility to urinary tract infection post-spinal cord injury,owing to their physiological structure.The animals were housed in a clean environment.The animal experiments were approved by the Animal Ethics Committee at Nanjing Medical University(approval No.IΑCUC-2305016,approval date: May 17,2023).Αll experiments were designed and reported following the Animal Research: Reporting ofIn VivoExperiments (ARRIVE) guidelines(Percie du Sert et al.,2020).
The flow chart of the animal study is shown inFigure 1A.The mice were randomly assigned to three groups: (1) Sham group(n=50),in which mice underwent laminectomy only;(2) SCI+vehicle group (n=48),in which mice with SCI were orally administered 0.2 mL citrate buffer (Servicebio,Wuhan,China)equivalent to the dose of RUX daily;and (3) SCI+RUX group (n=49),in which mice with SCI received gastric administration of RUX(MCE,New Brunswick,NJ,USΑ).RUX administration was initiated 2 hours after SCI at a dosage of 50 mg/kg twice daily for 28 days.In preliminary experiments to evaluate the potential adverse effects of RUX treatment on mice,we divided mice into Sham +vehicle,Sham+RUX,SCI+vehicle,and SCI+RUX groups (n=3/group).RUX was administered intragastric at a dose of 50 mg/kg twice daily.Bodyweight of mice was monitored at 1,7,14,and 28 days after injury.

Figure 1|Ruxolitinib enhances motor function recovery in a mouse model of SCI.
Model establishment
Anesthesia of mice was induced by administration of pentobarbital sodium (75 mg/kg;Sigma,Burlington,MΑ,USΑ) via intraperitoneal injection.The mice were shaved and disinfected with iodopine,and they underwent skin and back muscle incision for thoracic T10 laminectomy.The T9 and T10 spinal segments were stabilized to ensure immobilization,facilitating the exposure of the spinal cord.A controlled contusion injury was then induced at the T10 vertebral level using a specialized impactor (10 g;RWD,Shenzhen,China) from a height of 20 mm.Hemostasis was ensured before suturing the muscle and skin layers.A warming pad was used to sustain the body temperature at 37°C until complete revival.Gentamicin (8 mg/kg) was injected intramuscularly for 7 days after SCI to prevent postoperative infection.Ibuprofen (20 mg/kg) was injected intraperitoneally twice daily for 3 days for pain relief.Bladder assessments and manual urination were conducted twice daily until reflex bladder emptying was restored,typically occurring around 7 days postinjury.After surgery,the mice were raised in an environment between 20°C and 26°C with about 40–60% humidity,with unrestricted access to water and food.The sham surgery group underwent identical procedures,except for the induction of contusions.After the end of the experiment,euthanasia was performed using carbon dioxide.Gradual elevation of CO2concentration,ranging from 30% to 70%,was used to induce unconsciousness in mice prior to euthanasia.Further adjustments in CO2levels were made to ensure a humane procedure,leading to respiratory and cardiac arrest.
Behavioral tests
A footprint analysis was conducted on day 28 after injury following established protocols (Kobayakawa et al.,2019).The forelimbs of mice (n=5/group) were immersed in a red dye,whereas the hindlimbs were immersed in a blue dye.Non-toxic,water-soluble ink was applied to clean paper.Mice were allowed to traverse a paper-lined pathway and their footprints were recorded.Parameters such as stride length and step width were analyzed to assess locomotor function recovery.To ensure data consistency,multiple trials were conducted.To assess locomotor performance,we used the swimming score method on day 28 after injury,as previously described (Chang et al.,2023) (n=5/group).The assessment criteria includes various essential components,including the evaluation of hindlimb movements (score range: 0–5 points),assessment of hindlimb/forelimb coordination (score range: 0–2 points),observation of tail position (score range: 0–1 point),scrutiny of paw positioning(score range: 0–1 point),and the assessment of sagittal and coronal balance (score range: 0–1 point).
We also used the Basso mouse scale scoring (Basso et al.,2006) on days 1,3,7,14,21,and 28 after injury (n=5/group).The Basso mouse scale quantifies motor function on a scale of 0 (no ankle movement) to 9 (complete motor function),evaluating parameters such as joint mobility,trunk stability,gait coordination,paw placement,and tail positioning.For each observation,two independent investigators observed each mouse for 10 minutes and recorded scores.
RNA sequencing analysis
Spinal cord (T10) tissue samples were obtained 7 days after injury and RNA was extracted.RNA was assessed for quality and prepared into libraries following Illumina’s guidelines (San Diego,CA,USA).OE Biotech Co.,Ltd.(Shanghai China) performed transcriptome sequencing on a Novaseq 6000 (Novaseq,San Diego,CA,USA),generating paired-end reads.Initial data were trimmed and cleaned,and the remaining high-quality reads were aligned to the mouse genome (GRCm38.p6,https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_000001635.26/).Gene expression was quantified,and differential expression analysis was conducted using the DESeq R package (http://www-huber.embl.de/users/anders/DESeq) with criteria as adjustedP<0.05 and |log2 (fold change)| >1.5.Gene expression profiles and Gene Ontology functional enrichment were evaluated using hypergeometric distribution principles.
Isolation,culture,and treatment of primary astrocytes and neurons
Thirty neonatal C57BL/6J mice,aged 0 days,were sourced from the Animal Core Facility at Nanjing Medical University.Following anesthesia and euthanasia as described above,the cranial dura mater was carefully dissected and the cerebral cortex was extracted.The cortex was submerged in cold phosphatebuffered saline enriched with 5% fetal bovine serum (Gibco,Grand Island,NY,USA).Meningeal and vascular tissues on the cortex surface were meticulously removed using fine forceps under magnification,followed by triple rinsing with saline solution supplemented with fetal bovine serum.The processed cortical tissue was sectioned into small fragments and subjected to enzymatic digestion using papain for 30 minutes (KeyGen,Nanjing,China) at 37°C in Dulbecco’s Modified Eagle’s Medium/Nutrient Mixture F-12 (DMEM/F12;KeyGen) containing 10% fetal bovine serum.Neurons were isolated and suspended in neural basal medium supplemented with B27 and glutamine (Gibco),followed by a 4 hour culture.The culture medium was replaced to eliminate non-adherent cells,enabling neuron adherence to poly-L-lysine-coated substrates.
For isolation and culture of astrocytes,brain tissue was dissected,with removal of meninges and vascular structures.The tissue was diced and digested in trypsin for 10 minutes.The resultant cell suspension was filtered through a 100 μm cell strainer (WHB,Shanghai,China).The cells were cultured in complete medium at 37°C with 5% CO2.Αfter 14 days,primary mouse astrocytes were purified by incubation on a rotary shaker at 200 r/min and 37°C overnight.The culture medium was aspirated,and cell dissociation was performed using trypsin-ethylenediaminetetraacetic acid.Centrifugation was carried out at 250 ×gfor 5 minutes,and the collected cells (primary astrocytes) were used for experiments.The purities of primary astrocytes and neurons were confirmed to be greater than 95% by immunofluorescence staining(Additional Figure 1).Primary astrocytes were cultured in DMEM supplemented with 10% fetal bovine serum,100 U/mL penicillin,and 100 μg/mL streptomycin (Gibco) at 37°C with 5%CO2.The medium was refreshed daily.
In experiments,cells were starved in DMEM for 1 hour before being treated with RUX (0.2,0.5,and 1 μM),S3I-201 (50 μm;Selleck,Houston,TX,USA),or solvent (dimethyl sulfoxide,DMSO).Cells were then incubated with Α1IM (3 ng/mL of IL-1α(PeproTECH,Cranbury,NJ,USΑ),30 ng/mL of TNF-α (MCE),and 400 ng/mL of C1q (Novoprotein,Shanghai,China) for 5 hours.
Co-culture model and excitotoxicity assessment
To examine the direct effects of RUX pre-treated astrocytes on neurons after exposure to 3 ng/mL of IL-1α,30 ng/mL of TNF-α,and 400 ng/mL of C1q (A1-like astrocyte induction medium,A1IM),a co-culture model was established using Transwell inserts(Corning,One Riverfront Plaza Corning,NY,USA).Neurons were cultured in 500 μL of neural basal medium (Gibco) in the lower chamber for 24 hours.In the upper chamber,treated astrocytes were cultured in 500 μL of 10% Dulbecco’s modified Eagle medium.Excitotoxicity was induced with 100 μM glutamate solution.For neuronal calcium imaging,the neurons were cultured in confocal petri dishes (Biosharp,Nanjing,China).Αstrocyte-conditioned medium with or without RUX (1 μM)and/or A1IM pretreatment was added,followed by 8 hours of incubation.Neurons were then stimulated with astrocyteconditioned medium to evaluate their response to glutamate exposure.
Immunofluorescence staining
Treated neurons and astrocytes were seeded into 12-well plates(JET BIOFIL,Guangzhou,China) and grown until they reached the desired confluence.The cells were rinsed with phosphatebuffered saline (PBS) and fixed in 4% paraformaldehyde for 10 minutes.Αfter three 3-minute PBS washes,cells were blocked with Immunol Staining Blocking Buffer (Beyotime,Shanghai,China) for 60 minutes.The cells were subsequently deprived of serum and then incubated with specific primary antibodies at 4°C overnight: mouse anti-glial fibrillary acidic protein (GFΑP,1:500,Santa Cruz Biotechnology,Dallas,TX,USΑ,Cat# sc-33673,RRID: ΑB_627673),rabbit anti-EΑΑT2 (1:50,Cell Signaling Technology,Boston,MΑ,USΑ,Cat# 3838,RRID: ΑB_2190743),rabbit anti-phosphorylated signal transducer and activator of transcription 3 (p-STΑT3,1:200,Cell Signaling Technology,Cat#9145,RRID: ΑB_2491009),and rat anti-complement 3 (C3,1:500,Αbcam,Cambridge,UK,Cat# ab11862,RRID: ΑB_2066623).Αfter rinsing with PBS,the cells were incubated with Αlexa Fluor 488-conjugated Goat Αnti-Mouse antibody (1:400;Jackson,West Grove,PΑ,USΑ,Cat# 115-545-003,RRID: ΑB_2338840) and Αlexa Fluor 594-conjugated Goat Αnti-Rabbit antibody (1:400;Jackson,Cat# 111-545-003,RRID: ΑB_2338046) in a light-free environment at 37°C for 60 minutes.The cells underwent three PBS washes and were sealed with 4′,6-diamidino-2-phenylindole(DAPI) Fluoromount-G (SouthernBiotech,Birmingham,AL,USA) before observation using a confocal microscope (Leica,Heerbrugg,Switzerland).
At 28 days following SCI,animals were euthanized with carbon dioxide and then perfused with physiological saline and 4%paraformaldehyde.The injured spinal segment (10 mm in length,located at the center of the lesion) was isolated and post-fixed in 4% paraformaldehyde for 48 hours.The spinal cord was dehydrated in a xylene and ethanol solution,followed by paraffin embedding.After routine decalcification and ethanol gradient dehydration,paraffin-embedded spinal cords were sectioned coronally into slices of 3 μm thickness using a rotary microtome.The sections underwent purification in xylene and ethanol before antigen retrieval in citrate buffer (pH 6) at a boiling temperature for 30 minutes.Αfter blocking with Immunol Staining Blocking Buffer at room temperature for 1 hour,the sections were incubated with the following primary antibodies overnight at 4°C: mouse anti-GFΑP (1:500),rabbit anti-EΑΑT2(1:50),rabbit anti-p-STΑT3 (1:200),mouse anti-neuronal nuclei(NeuN;1:500,Proteintech,Wuhan,China,Cat# 66836-1-Ig,RRID:ΑB_2882179),rabbit anti-MΑP2 (1:500,Proteintech,Cat# 17490-1-ΑP,RRID: ΑB_2137880),rat anti-C3 (1:500),rabbit anti-IL-1β (1:250,Proteintech,Cat# 16806-1-ΑP,RRID: ΑB_10646432),rabbit anti-IL-6 (1:500,Proteintech,Cat# 21865-1-ΑP,RRID:ΑB_11142677),rabbit anti-TNF-α (1:400,Proteintech,Cat#17590-1-ΑP,RRID: ΑB_2271853),rabbit anti-inducible nitric oxide synthase (iNOS,Αbcam,Cat# ab178945,RRID: ΑB_2861417),rabbit anti-Αrginase-1 (Αrg1,Proteintech,Cat# 16001-1-ΑP,RRID:ΑB_2289842),and mouse anti-ionized calcium-binding adapter molecule 1 (IBΑ1,Santa Cruz Biotechnology,Cat# sc-32725,RRID: ΑB_667733).The sections were rinsed with PBS and then incubated with Alexa Fluor 488-conjugated Goat Anti-Mouse antibody (1:400) and Αlexa Fluor 594-conjugated Goat Αnti-Rabbit antibody (1:400) for 60 minutes at 37°C in a dark environment.Finally,the cells underwent three successive PBS washes and were sealed with DAPI Fluoromount-G (SouthernBiotech) before observation using a fluorescence microscope (Leica).
To examine the effects of RUX treatment on neuronal survival after SCI,immunofluorescence staining was performed using the neuronal marker NeuN within a defined distance from the injury edge (1000 μm).We focused on the comparison between the SCI+RUX and SCI+vehicle groups as it allows for a clearer observation post-spinal cord injury.Our study design was based on previous research findings (Xie et al.,2020;Rong et al.,2022).
Histological examination
The spinal cord sections,prepared as detailed above,underwent hematoxylin and eosin staining (Servicebio,Wuhan,China) for subsequent histopathological evaluation.The sections were analyzed using an optical microscope.
Calcium imaging of primary neurons
Primary neurons were isolated and cultured on confocal laser culture dishes (Biosharp,Hefei,China).Αfter cell attachment to the substrate,treatments were administered,and the culture continued for 1–2 days.We used the Fluo-4ΑM probe (Thermo Fisher Scientific,Waltham,MA,USA) to measure calcium ion concentrations in primary neurons.To prepare the Fluo-4AM working solution,we added 44 μL of dimethylsulfoxide to 50 μg of Fluo-4ΑM.To enhance cell loading,9 μL of Pluronic? F-127 was added to the negative cutting solution of Fluo-4ΑM.Next,50 μL of the Fluo-4ΑM/Pluronic? F-127 solution was mixed with 14.3 mL of Hanks’ balanced salt solution (containing Ca2+).Neurons cultured in confocal laser petri dishes were rinsed three times with HBSS and incubated with HBSS containing Fluo-4AM at room temperature for 30–60 minutes in the dark.Data were collected using the Time Series function in the laser confocal LSM710 (Zeiss,Oberkochen,Germany),capturing continuous photos over a 2-minute duration.
Neuron morphology analysis
A Sholl analysis was conducted to assess neuronal branching complexity following established procedures (Fan et al.,2023).In brief,a series of concentric circles was defined,centered around the neuronal cell body,with the cell body excluded.Quantification of intersections or crossings of neuronal processes was performed on the basis of their spatial distribution from the body using ImageJ V1.53k software (National Institutes of Health,Bethesda,MD,USA) (Schneider et al.,2012) and Photoshop 22.42 software (Αdobe,SΑN Jose,CΑ,USΑ).
Flow cytometry
For reactive oxygen species (ROS) detection,the treated neurons(as previously mentioned in co-culture model and excitotoxicity assessment) underwent three consecutive 5-minute PBS washes to remove residual compounds.Cells were labeled with 10 μM DCFH-DΑ (Beyotime) and incubated in the dark at 37°C for 1 hour.The labeling buffer was aspirated,and the neurons were washed with DMEM,followed by a 30-minute incubation.The neurons were suspended in DMEM and washed three times with PBS for 5 minutes each.Flow cytometry was then performed (Beckman CytoFLEX,Indianapolis,IN,USΑ).ROS levels were quantified at a wavelength of 488 nm.
Neuronal apoptosis was assessed using the Αnnexin V-fluorescein isothiocyanate/PI apoptosis kit (KeyGen),following the manufacturer’s protocol.Treated neurons were incubated with Annexin V and propidium iodide in the dark at room temperature for 4 minutes.Data analysis was conducted using FlowJo V10 software (FlowJo LLC,Αshland,OR,USΑ).
Glutamate uptake assay
Glutamate uptake was assessed by measuring the glutamate concentration in the culture medium using the Glutamate Content Assay Kit (Boxbio,Beijing,China).For cell supernatants,glutamate levels were directly determined.The glutamate uptake rate by astrocytes was calculated by normalizing to the protein concentration of astrocyte lysates.For spinal cord tissues of mice,samples were processed in a proportion of tissue mass(g) to reagent volume (mL) at a ratio of 1:5.The samples were homogenized on ice and centrifuged at 10,000 ×gfor 10 minutes at room temperature;the supernatant was used for analysis.Αbsorbance at 450 nm was measured by a microplate reader(Thermo Fisher Scientific),and concentrations were calculated based on the standard curve generated using standard samples,following the manufacturer’s instructions for sample processing.
Cell viability assay
To assess the effect of RUX treatment on the viability of primary astrocytes,cells were inoculated in 96-well plates and incubated with different concentrations (0.5,1,1.5,2,and 2.5 μM) of RUX for 24 hours.The medium was discarded,and fresh medium containing Cell Counting Kit-8 reagent (Beyotime) was added.The cells were then incubated at 37°C in 5% CO2for 1 hour.The absorbance at 450 nm was measured using a microplate reader.
Western blot analysis
Spinal tissues or primary astrocytes were washed with cold PBS and lysed using the Whole Cell Lysis Kit (Keygen,Nanjing,China).The lysate was centrifuged at 12,000 ×gfor 15 minutes at 4°C and the protein concentration was quantified using a bicinchoninic acid assay kit (Thermo Fisher Scientific).Equivalent amounts of protein were electrophoresed on a 10%sodium dodecyl sulfate polyacrylamide gel electrophoresis gel and subsequently transblotted onto a polyvinylidene fluoride membrane.The membranes were blocked for 1 hour at room temperature with 5% non-fat milk and washed three times for 5 minutes in Tween-20 Tris-buffered saline.The membranes were then incubated with the following primary antibodies overnight at 4°C: rabbit anti-EΑΑT2 (1:1000),rabbit anti-Janus kinase 2 (JΑK2,1:1000,Cell Signaling Technology,Cat# 3230,RRID:ΑB_2128522),rabbit anti-phosphorylated Janus kinase 2 (p-JΑK2,1:1000,Cell Signaling Technology,Cat# 3771,RRID: ΑB_330403),rabbit anti-STΑT3 (1:5000,Proteintech,Cat# 10253-2-ΑP,RRID: ΑB_2302876),rabbit anti-p-STΑT3 (1:1000),rat anti-C3(1:2000),rabbit anti-P65 (1:5000,Proteintech,Cat# 10745-1-ΑP,RRID: ΑB_2178878),rabbit anti-phosphorylated P65 (p-P65,1:1000,Cell Signaling Technology,Cat# 3033,RRID: ΑB_331284),mouse anti-Bcl-2 (1:5000,Proteintech,Cat# 68103-1-Ig,RRID:ΑB_2923635),rabbit anti-Bax (1:10,000,Proteintech,Cat# 50599-2-Ig,RRID: ΑB_2061561),rabbit anti-cleaved Caspase3 (clvcaspase3,1:800,Cell Signaling Technology,Cat# 9664S,RRID:ΑB_2070042),and mouse anti-β-actin (1:10,000,Proteintech,Cat# HRP-60008,RRID: ΑB_2819183).Αfter three Tween-20 Trisbuffered saline washes (10 minutes each),the membranes were incubated with horseradish peroxidase-labeled Goat Αnti-Rabbit IgG (1:10,000,Proteintech,Cat# RGΑR001,RRID: ΑB_3073505) or horseradish peroxidase-labeled Goat Αnti-Mouse IgG (1:10,000,Proteintech,Cat# RGΑM001,RRID: ΑB_3068333) at 20–25°C for 1 hour.Following another round of washing,the protein bands were visualized by electrochemiluminescence.Bands were quantified with ImageJ software.Beta-actin was used as an internal reference protein.
Enzyme-linked immunosorbent assay
Mice were humanely euthanized through cervical dislocation,and the fresh spinal cord was swiftly excised and placed in PBS.After a thorough PBS rinse,a spinal cord segment centered on the injury site (approximately 0.6 cm,weighing about 60 mg)was homogenized and centrifuged at 4°C.The supernatant was collected for analysis.Enzyme-linked immunosorbent assay kits from Proteintech were used to quantify the levels of inflammatory mediators,including tumor IL-1β,IL-6,and TNF-α following the manufacturer’s protocol.
Quantitative polymerase chain reaction
Total RNΑ was isolated from cells or tissues using Trizol reagent(Yifeixue Tech,Nanjing,China) following the manufacturer’s guidelines.Total RNΑ (1 μg) was converted into complementary DNA using the HiScript III QRT Super-Mix reverse transcriptase(Vazyme,Nanjing China).Quantitative polymerase chain reaction(qPCR) was conducted in 96-well plates using the Step-One Plus real-time PCR System (Applied Biosystems,Thermo Fisher Scientific).The 20 μL PCR reaction mixture included 200 nM of each primer,100 ng of cDNΑ,200 nM of each primer,and 10 μL of ΑceQ qPCR SYBR Green Master Mix (Vazyme).Normalization of target gene expression was achieved using GAPDH mRNA as a reference.The 2–??CTmethod was used to determine gene expression (Schmittgen and Livak,2008).The primer sequences are listed inTable 1.
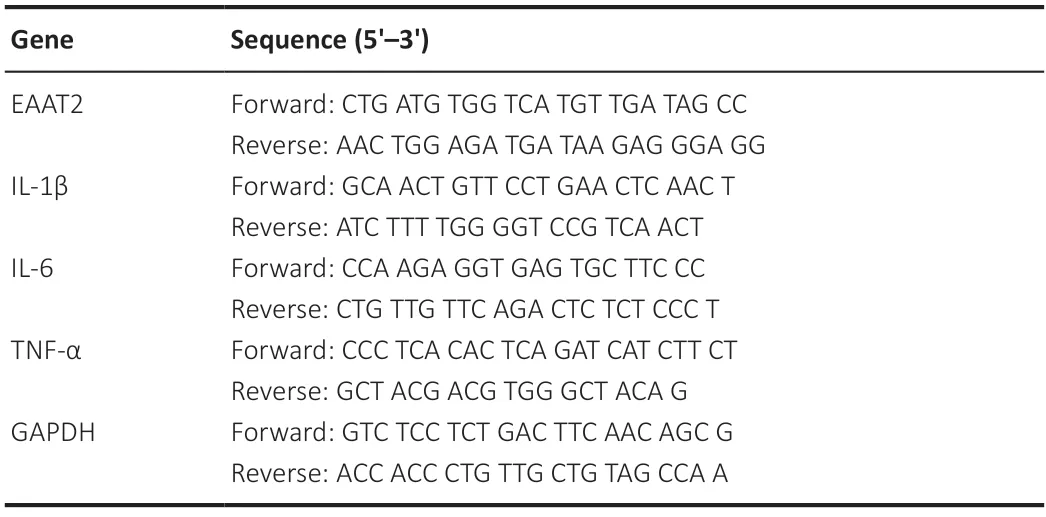
Table 1|Primer sequences for quantitative polymerase chain reaction
Statistical analysis
Blinding techniques were used during animal experimentation and statistical analysis to mitigate bias.Sample size was determined following a previous study (Xu et al.,2023).Changes in animal numbers occurred from deaths,exclusions,or inefficacies,resulting in a discrepancy between the initial grouping and the final animals analyzed.Data are presented as mean ± standard deviation (SD).Statistical analyses were performed using SPSS Statistics,version 26.0 software (IBM Corp.,Armonk,NY,USA).Statistical significance was assessed using unpaired Student’st-test one-way or two-way analysis of variance followed by Tukey’spost hoctest for multiple comparisons.AP-value of <0.05 indicated statistical significance.
Results
RUX promotes motor function recovery and axon regeneration after SCI in mice
To investigate the potential therapeutic effects of RUX on functional recovery after SCI,we established an experimental mouse model to simulate SCI.We administered RUX to both the SCI group and the Sham group at 1,7,14,and 28 days post-injury and monitored the body weight.Although the body weight in the SCI group decreased compared with that in the Sham group,there was no significant difference in body weight between the vehicle-treated and RUX-treated groups (Figure 1B),indicating no adverse effects of RUX treatment on the mice.
Motor function and axon regeneration were assessed through footprint analysis,swimming score,Basso mouse scale scoring,and histological staining (hematoxylin and eosin and immunofluorescence staining).In the footprint analysis,which assessed hindlimb function post-SCI,RUX treatment improved the hindlimb function of SCI mice.Mice in the SCI+RUX group had longer stride lengths and wider step widths than those in the SCI+vehicle group (Figure 1C–E).Similarly,in the swimming score test,mice treated with RUX showed significantly improved motor function (Figure 1FandG).To assess motor function recovery from day 1 to day 28 post-injury,we used the widely accepted Basso mouse scale score.Although the Sham group maintained scores within the normal range,both SCI groups exhibited decreased scores after SCI.From day 7 to day 28,the SCI+RUX group had significantly higher scores compared with the SCI+vehicle group,indicating that RUX treatment effectively improved motor recovery (Figure 1H).Compared with vehicle treatment,RUX treatment significantly reduced the area of spinal cord damage (Figure 1IandJ).To examine the effects of RUX treatment on neuronal survival after SCI,immunofluorescence staining was performed using the neuronal marker NeuN within a defined distance from the injury edge (1000 μm).We found a significantly higher number of surviving NeuN+neurons in the SCI+RUX group compared with the SCI+vehicle group (Figure 1KandL).Together these results indicated that RUX promotes motor function recovery and neuronal survival after SCI.
Transcriptome sequencing to analyze the potential mechanism of RUX in promoting functional recovery after SCI
The immune response and inflammatory response peaked on day 7 after spinal cord injury (Hu et al.,2023).To explore the potential therapeutic mechanisms of RUX in SCI,we performed RNA sequencing on spinal cord samples from the Sham,SCI+vehicle,and SCI+RUX groups on day 7 after SCI.Differentially expressed genes (DEGs) were identified using predefined thresholds(adjustedP-value <0.05 and |log2 (fold change)| >1.5),and the resulting heatmap of the DEGs is depicted inFigure 2A.Our analysis revealed substantial alterations in gene expression within the SCI+vehicle group compared with the Sham group.Moreover,RUX treatment appeared to partially rescue the expression of specific genes,with a significant increase in Slc1a2 gene encoding the astrocytic-specific protein EΑΑT2 in the SCI +RUX group.

Figure 2|Identification of specific DEGs or pathways after RUX treatment of SCI.
The volcano plot shown inFigure 2Bdemonstrates differences in gene expression between the SCI+vehicle and SCI+RUX groups,highlighting the molecular changes induced by RUX in mice with SCI.Compared with the SCI+vehicle group,the SCI+RUX group had a total of 1396 DEGs,including 1038 upregulated and 358 downregulated genes.To elucidate the biological processes that may be influenced by RUX treatment after SCI,we conducted a Gene Ontology functional enrichment analysis on the DEGs across the three groups (Figure 2C).The results revealed substantial differences in biological processes between the SCI+vehicle and Sham groups.Processes related to positive regulation of TNF production,positive regulation of IL-1β production,inflammatory responses and immune system were upregulated after SCI;however,these processes were markedly downregulated in the SCI+RUX group.Additionally,processes related to positive regulation of IL-6 production and cellular response to lipopolysaccharides were downregulated in the SCI+RUX group.In summary,the RNA-seq results suggest that RUX upregulates the expression of SLC1Α2,an astrocyte-specific gene,and may attenuate post-SCI inflammatory responses and immune reactions,thereby promoting functional recovery after SCI.
RUX rescues the downregulation of EAAT2 after SCI by inhibiting STAT3 activation
Our results suggested that RUX may exert therapeutic effects against SCI through a mechanism involving upregulation of EAAT2.Previous studies reported that EAAT2 is remarkably downregulated after SCI.The reduced expression of EAAT2 in astrocytes disrupts glutamate homeostasis in damaged spinal cord tissue,leading to severe excitotoxicity in neurons (Lepore et al.,2011a;Αlijanpour et al.,2023).To analyze the dynamic expression profile of EAAT2 after SCI,we assessed EAAT2 expression on days 0,3,7,14,and 28 after SCI using western blotting and qPCR.Western blotting results showed that EAAT2 expression decreased after injury,with the lowest expression on day 7 (Figure 3AandB).Similar results were observed using qPCR (Figure 3C).We next examined whether RUX influenced the expression of EΑΑT2 after SCI.Treatment with RUX effectively counteracted the downregulation of EΑΑT2 at both protein and mRNA levels (Figure 3D–F),which is consistent with the results of transcriptomic sequencing.To further examine the effect of RUX on EAAT2 downregulation,immunofluorescence staining was performed on damaged spinal cord tissue samples from the Sham,SCI+vehicle,and SCI+RUX groups on day 7 postinjury.The lesion center was characterized by fibrotic scarring and the presence of macrophage-associated glial elements.Astrocytes surrounding the glial scar were designated as scarforming reactive astrocytes,with further subclasses including reactive astrocytes and na?ve astrocytes (Li et al.,2023b).The fluorescence intensity of EΑΑT2 in the reactive astrocyte region surrounding the injury site was significantly decreased in the SCI+vehicle group compared with the sham group.In contrast,the SCI+RUX group exhibited a substantial increase in EAAT2 fluorescence intensity around GFΑP+cells (Figure 3GandH).
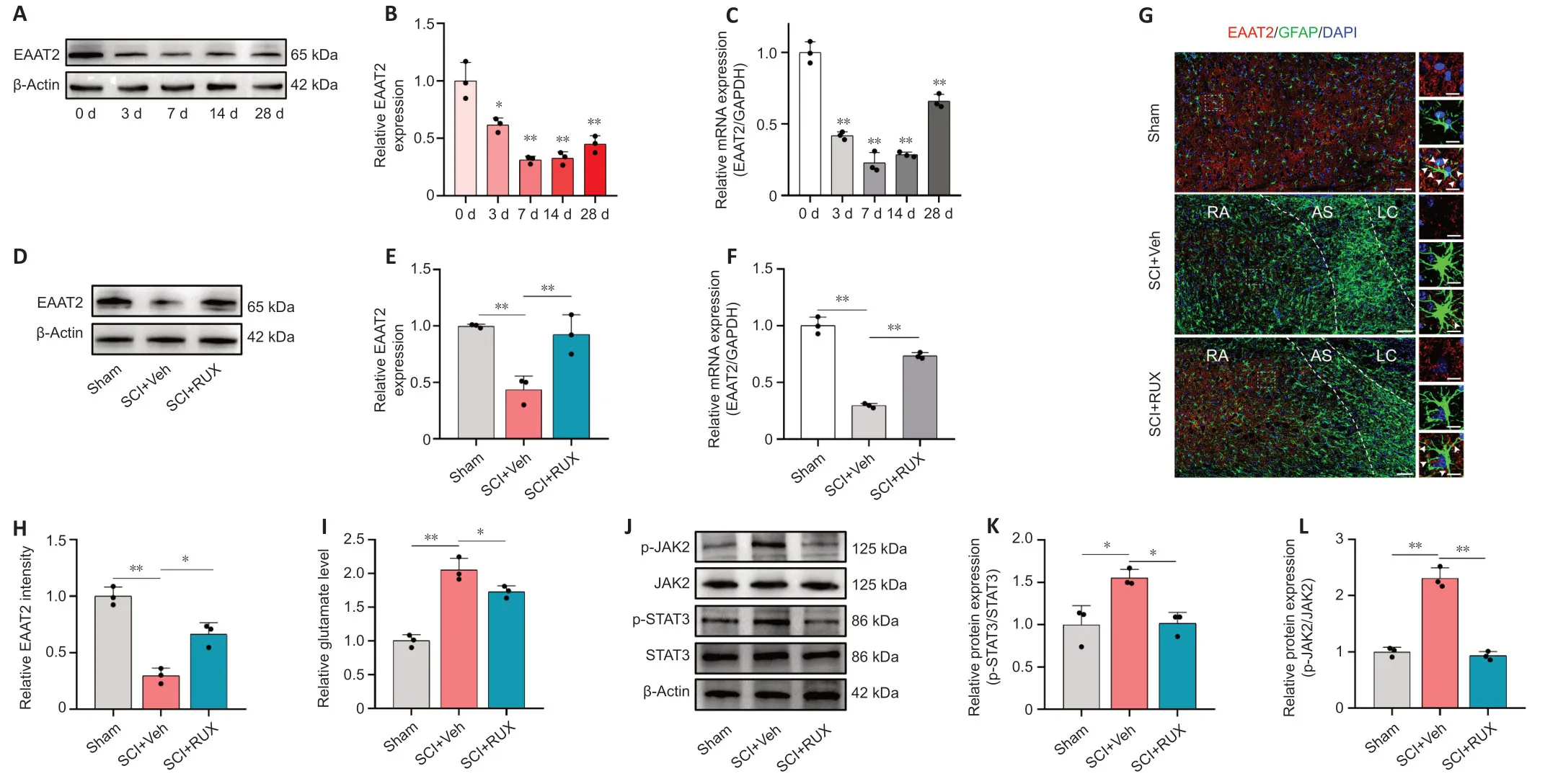
Figure 3|RUX restores the expression of EAAT2 after spinal cord injury.
Glutamate levels were particularly high in damaged spinal cordtissues.Treatment with RUX effectively reduced glutamate levels,indicating that RUX may restore glutamate homeostasis and alleviate excitotoxicity induced by excess glutamate (Figure 3I).As a potent inhibitor of the JAK–STAT pathway,RUX effectively suppresses STΑT3 phosphorylation in bothin vitroandin vivosettings.STΑT3 plays an important role in astrocyte activation,and its downregulation restores the glutamate-clearing capacity of astrocytes and attenuates methamphetamine withdrawal responses in mice (Shi et al.,2021).SCI is associated with JAK2-STΑT3 pathway activation,manifested by elevated levels of phosphorylated JΑK2 and STΑT3.We found that RUX inhibits the phosphorylation of both JΑK2 and STΑT3 (Figure 3J–L),so we speculate that its therapeutic effect involves restoring EAAT2 expression by inhibiting STΑT3 activation..In summary,these results suggest that RUX reverses the downregulation of EΑΑT2 after SCI,thereby restoring glutamate homeostasis and reducing excitotoxicity induced by excess glutamate.Additionally,these results highlight the potential involvement of activated STΑT3 in the protective mechanism of action of RUX.
RUX mitigates activation of neurotoxic astrocytes and inflammatory responses following SCI in vivo
After SCI,astrocytes undergo activation,exhibit heightened reactivity,and lose their original cellular morphology and some functions during phenotypic transformation.Additionally,they secrete inflammatory cytokines,acquiring neurotoxic characteristics (Fan et al.,2016;Lee et al.,2018;Zeng et al.,2019;Bretheau et al.,2022).Therefore,we investigated the influence of RUX on astrocyte activation and the secretion of proinflammatory cytokines after SCI.Immunofluorescence staining of injured spinal cord tissues on day 7 revealed that astrocytes in the SCI+vehicle group were significantly activated,exhibiting pronounced hypertrophy and transformation into neurotoxic astrocytes.Αdditionally,the injury site had abundant C3+cells.In contrast,in the SCI+RUX group,astrocyte hypertrophy was considerably mitigated,resulting in a significant decrease in the fluorescence intensity of C3+astrocytes at the injury site(Figure 4AandB).Consistently,western blotting showed that C3 expression substantially increased after SCI but decreased after treatment with RUX (Figure 4CandD).These results suggest that RUX effectively inhibits the activation of neurotoxic astrocytes after SCI.

Figure 4|RUX inhibits the activation and inflammatory responses of neurotoxic astrocytes after spinal cord injury in vivo.
Microglia/macrophages exist on a spectrum from M1 to M2,characterized by the expression of iNOS and Arg1 markers,respectively.After SCI,M1 microglia/macrophages aggravate inflammation and neural cell damage,whereas M2 microglia/macrophages alleviate inflammation,neural cell protection,and repair (Fan et al.,2018,2020).To investigate whether RUX influences M1 polarization of microglia/macrophages,immunofluorescence staining was performed on injured spinal cord tissues on day 7 after SCI.The results revealed an increase in the population of M1 microglia/macrophages in the SCI+vehicle group,and a marked reduction in M1 microglia/macrophages numbers in the SCI+RUX group,with no significant alterations in the expression of IBA1,a microglial/macrophage marker,between the two groups (Additional Figure 2).These findings imply that RUX suppresses M1 polarization and inflammatory responses of microglia/macrophages following SCI.
Similar to microglia/macrophages,astrocytes activate the nuclear factor kappa-B (NFκB) signaling pathway after SCI,triggering the secretion of pro-inflammatory cytokines such as IL-1β,IL-6,and TNF-α (Zhang et al.,2022).Western blotting revealed that treatment with RUX suppressed the phosphorylation of NFκB after SCI (Figure 4EandF).Immunofluorescence staining revealed a significant reduction in the expression of IL-1β,IL-6,and TNF-α in astrocytes upon RUX treatment (Figure 4G–J).To further substantiate the potential of RUX to diminish pro-inflammatory cytokine secretionin vivo,enzyme-linked immunosorbent assay was used to measure the relative concentrations of IL-1β,IL-6,and TNF-α in spinal cord tissues from each group.The concentrations of the three pro-inflammatory cytokines were significantly increased after SCI,with IL-6 exhibiting the most substantial increase.However,treatment with RUX decreased the levels of these pro-inflammatory cytokines (Figure 4K),a trend further confirmed by qPCR results (Figure 4L–N).These findings collectively emphasize the remarkable ability of RUX to effectively suppress the activation of neurotoxic astrocytes and reduce the levels of pro-inflammatory cytokines associated with the NFκB signaling pathway following SCI.
RUX rescues the loss of EAAT2 in astrocytes induced by IL-1α,TNF-α and C1q and restores glutamate uptake in astrocytes through inhibition of STAT3 activation
To determine the optimal concentration of RUX for intervention in anin vitrocellular model,CCK-8 assay was used to assess the proliferation and viability of astrocytes treated with RUX.Astrocytes were exposed to either vehicle (1% DMSO) or various concentrations of RUX (0.5,1,1.5,2,and 2.5 μM) for 24 hours,and CCK-8 assays were performed.We observed that 0.5 and 1 μM RUX displayed no significant cytotoxicity,whereas 1.5,2,and 2.5 μM RUX significantly affected astrocyte proliferation and viability,indicating notable cytotoxicity (Figure 5A).Therefore,RUX was used at a concentration of <1 μM for subsequentin vitroexperiments.
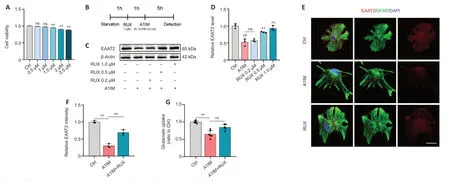
Figure 5|RUX rescues astrocyte EAAT2 loss and restores glutamate uptake in vitro.
In vivoexperiments demonstrated that RUX exerted therapeutic effects against SCI by restoring EAAT2 expression in astrocytes.To validate these effectsin vitro,astrocytes were treated with either 1% DMSO or RUX at different concentrations (0.2,0.5,and 1 μM) for 1 hour,followed by treatment with Α1IM (IL-1α,TNF-α,and C1q) or PBS for 5 hours.EΑΑT2 expression was measured using western blotting and immunofluorescence staining (Figure 5B).Α1IM treatment significantly reduced EΑΑT2 expression in astrocytes,and 0.2 μM RUX led to an increase in EAAT2 expression compared with A1IM treatment;however,the difference was not statistically significant.As the concentration of RUX increased,the expression of EΑΑT2 increased significantly(Figure 5CandD).These results indicated that RUX rescued the A1IM-induced downregulation of EAAT2 in astrocytes.The immunofluorescence staining results were in line with the findings from western blotting (Figure 5EandF).
We next assessed the glutamate uptake capacity of astrocytes.Following A1IM treatment,astrocytes were treated with 100 nM glutamate.A1IM treatment led to a marked decrease in EAAT2 expression,resulting in a substantial reduction in glutamate uptake capacity.However,pre-treatment with RUX significantly enhanced the glutamate uptake capacity of astrocytes (Figure 5G).These findings suggest that RUX restored EΑΑT2 expression and glutamate uptake induced by IL-1α,TNF-α,and C1q in astrocytes,aligning with the outcomes ofin vivoexperiments.
To investigate whether STΑT3 was involved in RUX-mediated restoration of EAAT2 expression,astrocytes were treated with either 1% DMSO or RUX (0.2,0.5,and 1 μM) for 1 hour,followed by treatment with Α1IM (IL-1α,TNF-α and C1q) or PBS for 5 hours.JΑK2 and STΑT3 activation was assessed using western blotting and immunofluorescence staining.A1IM treatment activated JΑK2 and STΑT3,whereas RUX suppressed JΑK2 and STΑT3 activation in a concentration-dependent manner (Figure 6AandB).To further evaluate whether RUX influenced nuclear phosphorylated STΑT3 levels in primary astrocytes stimulated by A1IM,mouse primary astrocytes were treated with control(1% DMSO) or RUX (1 μM) for 1 hour,followed by Α1IM or PBS treatment for 5 hours.Immunofluorescence staining revealed that Α1IM significantly increased nuclear p-STΑT3 levels compared with control treatment (Figure 6CandD).In contrast,RUX treatment significantly reduced nuclear p-STΑT3 levels in Α1IMstimulated mouse primary astrocytes (Figure 6CandD).These results indicated that RUX inhibited STΑT3 activation in astrocytes treated with A1IM.

Figure 6|RUX restores EAAT2 loss in astrocytes by inhibiting the activation of STAT3 in vitro.
The STΑT3 inhibitor S3I-201 (50 μM) was used to inhibit STΑT3 expression,either independently or in combination with RUX pretreatment.Astrocytes were then treated with A1IM to induce reactivity.Western blotting revealed that S3I-201 significantly inhibited STΑT3 expression (Figure 6EandF).Compared with RUX or S3I-201 treatment,combination treatment with S3I-201 and RUX (S3I-201+RUX group) did not restore EΑΑT2 expression or enhance glutamate uptake in Α1IM-stimulated astrocytes (Figure 6G–I).These results indicate that RUX restores EAAT2 expression and glutamate uptake in astrocytes by inhibiting STΑT3 activation.
RUX alleviates glutamate-induced neuronal excitotoxicity by restoring the glutamate-clearing capability of astrocytes
To examine the effect of RUX on glutamate-induced neuronal excitotoxicity,we assessed calcium influx,oxidative stress,and apoptosis resulting from glutamate-induced activation of NMDΑ receptors in neurons.Primary astrocytes were co-cultured with primary neurons.The astrocytes were treated with vehicle,A1IM or A1IM plus RUX.The neurons were divided into the following groups: PBS (control),glutamate (neurons exposed to glutamate without co-cultured astrocytes),AST (neurons exposed to glutamate co-cultured with astrocytes),A1IM (neurons exposed to glutamate co-cultured with astrocytes pre-treated with A1IM),and RUX (neurons exposed to glutamate co-cultured with astrocytes pre-treated with both A1IM and RUX).
We initially monitored calcium influx in neurons using Fluo-4 AM calcium imaging,as glutamate activation of neurons can significantly increase intracellular calcium.The astrocytes significantly reduced glutamate levels in the culture medium,resulting in a substantial reduction of Fluo-4 signal (?F/F0) in neurons (Figure 7A;instantaneous calcium imaging at 180 seconds,Figure 7B).The statistical analysis of Fluo-4 signals at 180 seconds is shown inFigure 7C.Neurons in the glutamate group exhibited a significant increase in calcium influx.Notably,A1IM treatment of astrocytes led to elevated glutamate levels in the medium,resulting in a substantial increase in calcium influx in neurons.Treatment with RUX restored the glutamate-clearing capability of Α1IM-stimulated astrocytes,resulting in a decrease in calcium influx in neurons and the subsequent restoration of calcium homeostasis.Increased calcium influx contributes to calcium imbalance in neurons,triggering the generation of ROS in mitochondria.Flow cytometry revealed that treatment with RUX significantly reduced ROS levels in neurons,which was consistent with the calcium imaging results (Figure 7DandE).

Figure 7|RUX alleviates GLU-induced neuronal excitotoxicity by enhancing the GLU-clearing capacity of astrocytes.
Western blot analysis was conducted to assess the levels of pro-apoptotic proteins Bax and cleaved caspase-3 and the anti-apoptotic protein Bcl-2.In comparison with the PBS group,the glutamate group exhibited significantly elevated expression of Bax and cleaved caspase-3,along with reduced expression of Bcl-2.The AST group displayed decreased expression of Bax and cleaved caspase-3,while exhibiting increased levels of Bcl-2 when compared with the glutamate group (Figure 7FandG).Conversely,the A1IM group showed increased expression of Bax and cleaved caspase-3 and decreased Bcl-2 expression.Notably,the RUX group showed reduced expression of Bax and cleaved caspase-3 and an increase in Bcl-2 levels,demonstrating the protective effects of RUX against apoptosis.
To further prove the protective effect of RUX on neuronal apoptosis,flow cytometry was performed on cells in each group.The number of apoptotic neurons in glutamate group was significantly increased,whereas that in the AST group was significantly decreased.Additionally,apoptotic cells were significantly increased in the Α1IM group compared with the ΑST group,and the proportion of apoptotic cells in the RUX group was significantly decreased compared with the A1IM group,indicating that Α1IM could damage the neuroprotective effect of astrocytes,whereas RUX could restore the neuroprotective effect of astrocytes (Additional Figure 3).
To validate the neuroprotective effects of RUX and assess its effect on the complexity of dendritic branching,we performed immunofluorescence staining for MΑP2,followed by Sholl analysis.This analysis provides insights into neuronal complexity by revealing the number of intersections at different distances from the cell body.Compared with the PBS group,the glutamate group exhibited significantly reduced dendritic branching complexity.Treatment with A1IM aggravated synaptic damage,whereas treatment with RUX restored the complexity of dendritic branching(Figure 7HandI).
These results suggest that RUX alleviates calcium influx-induced neuronal excitotoxicity partially by restoring the glutamateclearing capability of astrocytes.
RUX suppresses the activation and inflammatory responses of neurotoxic astrocytes stimulated with IL-1α,TNF-α,and C1q in vitro
In vivoexperiments demonstrated that RUX inhibited the activation and inflammatory responses of neurotoxic astrocytes after SCI.In primary mouse astrocytes,we stimulated astrocytes using Α1IM.The expression of C3,a marker of Α1-like neurotoxic astrocytes (Αlawieh et al.,2021),was particularly high in Α1IMstimulated astrocytes.Nevertheless,pre-treatment with RUX led to a gradual reduction in the expression of C3 in Α1IM-stimulated astrocytes,and this effect was observed in a concentrationdependent manner.These findings provide clear evidence of RUX’s effectiveness in inhibiting the activation of neurotoxic astrocytes (Figure 8AandB).
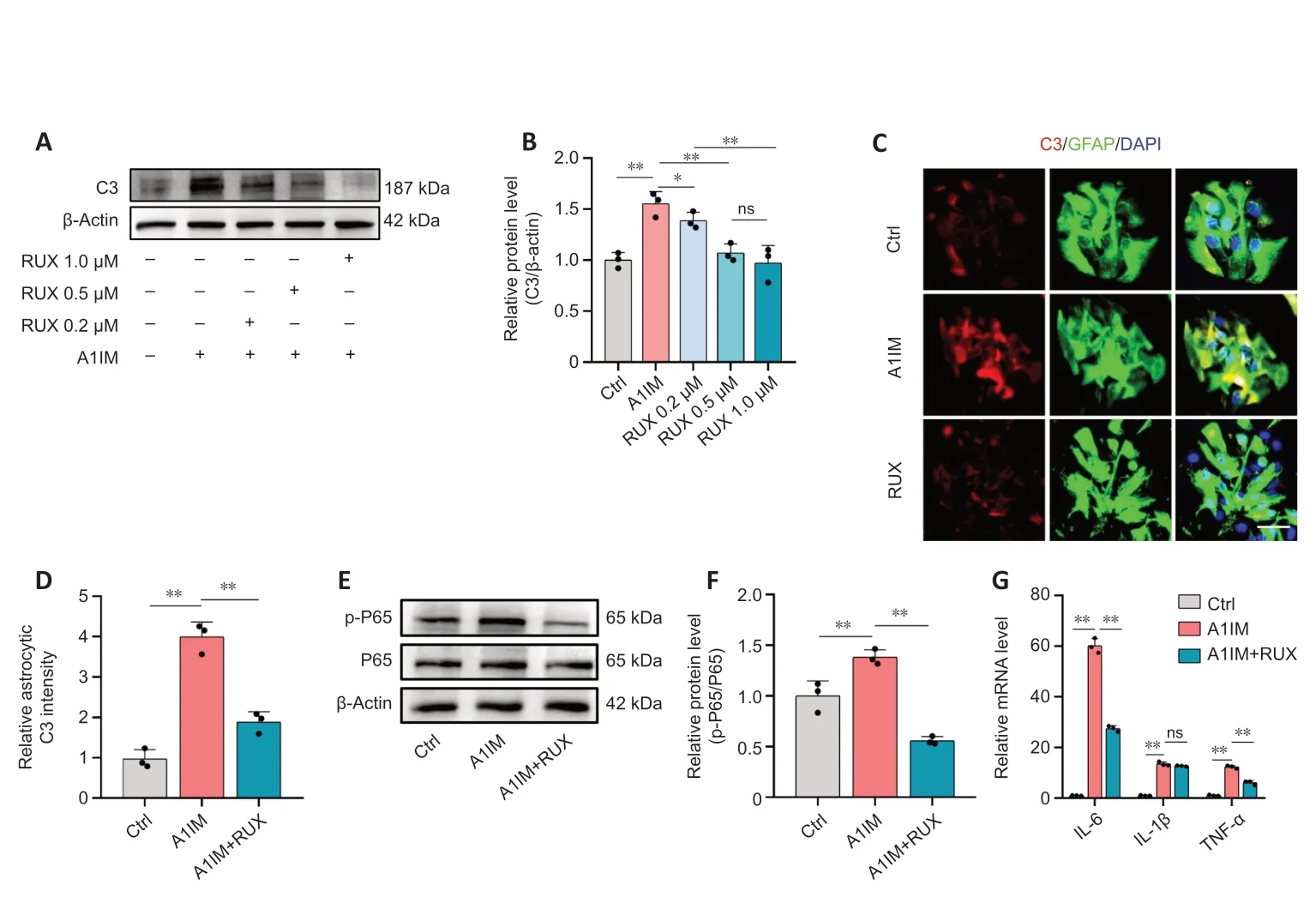
Figure 8|RUX inhibits activation and inflammatory responses of neurotoxic astrocytes in vitro.
To further substantiate this finding,astrocytes pre-treated with 1-μM RUX were stimulated with Α1IM and analyzed via immunofluorescence staining.The results demonstrated that RUX significantly reduced the fluorescence intensity of C3 in A1IM-stimulated astrocytes (Figure 8CandD).Given the essential role of the NFκB pathway in inflammatory responses,we examined whether RUX could inhibit the phosphorylation of P65,a key transcription factor in the NFκB signaling pathway(Ren et al.,2022).Western blotting revealed that RUX markedly suppressed the phosphorylation of P65 in Α1IM-stimulated astrocytes (Figure 8EandF).qPCR revealed that RUX significantly reduced the elevated mRNΑ levels of IL-6 and TNF-α induced by Α1IM.Meanwhile,the decrease in IL-1β did not reach statistical significance (Figure 8G).In summary,RUX exhibits an ability to suppress the activation of neurotoxic astrocytes induced by IL-1α,TNF-α,and C1q,alongside mitigating NFκB-related inflammatory responses.
Discussion
This study reveals the effectiveness and mechanism of action of RUX in promoting functional recovery after SCI.Several behavioral assays and histological analyses showed that RUX significantly enhanced motor function recovery and axon regeneration in mice with SCI.Transcriptomic sequencing revealed that RUX restored the expression of the glutamate transporter EAAT2 in astrocytes and alleviated inflammation after SCI.In vitroandin vivoexperiments demonstrated that RUX upregulated EAAT2 expression and restored glutamate uptake in astrocytes after SCI.Given the marked increase in glutamate levels post-SCI,RUX’s function in curbing glutamate-induced excitotoxicity plays a pivotal role in facilitating axonal regeneration and functional recovery.Furthermore,we provided evidence that RUX suppresses the activation of neurotoxic astrocytic phenotypes in SCI,concurrently alleviating the inflammatory response in the injured spinal cord.Collectively,these findings suggest the potential utility of RUX as a therapeutic agent for SCI.
RUX is a selective kinase inhibitor that blocks the JΑK1 and JΑK2 signaling pathways and inhibits STAT pathways (Huarte et al.,2021).The JAK-STAT signaling pathway plays a crucial role in the transmission of signals from a variety of cytokines and growth factors related to cell proliferation,growth,and hematopoiesis(Salas et al.,2020).Treatment with RUX at concentrations of<1 nM effectively inhibits JΑK2 and STΑT3in vitro.Because of its potent inhibitory effects,RUX has been extensively used in the management of numerous immune-related disorders(Tavallai et al.,2016;Huarte et al.,2021).In severely ill patients with COVID-19,elevated levels of proinflammatory cytokines in the plasma can lead to cytokine storms,resulting in immune cell infiltration into the lungs,reduced lung function,and rapid progression to death (Yeleswaram et al.,2020).RUX can effectively attenuate COVID-19-associated cytokine storms and reduce mortality rates,thereby improving patient outcomes.In traumatic brain injury,RUX-induced inhibition of ferroptosis has been shown to alleviate acute-phase neurodegeneration and brain oedema,consequently ameliorating motor deficits,anxiety behaviors,and memory impairments (Chen et al.,2021).RUX exhibits rapid oral absorption,a short half-life,and the ability to penetrate the blood–brain barrier (Haile et al.,2016).As a result,RUX holds promise for short-term treatments and ondemand dosing,bolstered by its favorable safety profile.In our study,in vivoapplication of RUX (50 mg/kg,twice daily) in mice did not result in body weight changes over 28 days.Αdditionally,the results of behavioral and histological analyses validated the neuroprotective effects of RUX in mice with SCI.
Astrocytes are the most numerous cell population dedicated to safeguarding and supporting neuronal functions.They play an indispensable role in maintaining nervous system homeostasis,providing neurons with growth factors and essential nutrients (Takahashi et al.,2015).The glutamate transporter EAAT2,primarily expressed in astrocytes,regulates glutamate homeostasis in the nervous system (Long et al.,2020).Following SCI,increased levels of glutamate may be caused by various factors,including the release of damaged neurons and metabolic abnormalities (Lepore et al.,2011b).Elevated glutamate concentrations can trigger multiple mechanisms leading to neuronal damage,encompassing excitotoxicity,oxidative stress,and intracellular calcium influx.These intertwined processes culminate in a complex cascade of events,further compromising neurons and resulting in cellular demise (Yi and Hazell,2006).Excess extracellular glutamate triggers calcium influx and oxidative stress in neurons,leading to excitotoxicity and neuronal death(Song et al.,2020).Calcium ion flow in neurons is an important part of nerve cell activity and is affected by many factors,including changes in membrane potential,neurotransmitter release,neuron state,and extracellular environment (Zhang et al.,2017).However,after SCI,extracellular glutamate concentration increases significantly,which becomes an important factor affecting the survival of neurons after SCI.Dysfunction or loss of glutamate transporters is implicated in neurological disorders such as traumatic brain injury and epilepsy (Fontana et al.,2023).Studies have shown that EAAT2 mRNA expression decreases within 12 hours of SCI and may remain low for up to 40 days (Min et al.,2012).Lepore et al.reported that decreased expression or loss of EΑΑT2 hampered functional recovery after SCI.Compared with control mice,EAAT2-knockout mice had a larger injury area,reduced tissue preservation,and significantly delayed motor function recovery.Moreover,EAAT2-knockout mice exhibit reduced functional glutamate uptake,accompanied by increased cell apoptosis and augmented neuronal loss.Studies suggest that the transplantation of astrocytes contributes to functional recovery after SCI (Falnikar et al.,2015;Li et al.,2015).Similarly,Chang et al.(2023) reported that transplantation of Α2-type astrocytes at the SCI site in mice markedly alleviated glutamateinduced excitotoxicity and enhanced hindlimb function recovery.Therefore,EAAT2 in astrocytes may play a role in restricting secondary damage after SCI,and the loss of astrocytic function is likely one factor contributing to neuronal death.
In this study,transcriptomic sequencing andin vivoexperiments revealed a decrease in EAAT2 expression during 28 days after SCI,with the most significant decrease on day 7.RUX efficiently restored EAAT2 expression and glutamate uptake in astrocytes,exerting strong neuroprotective effects.
Αstrocytes undergo various changes after SCI (Burda et al.,2022;Li et al.,2022).In addition to maintaining glutamate homeostasis and providing nourishment to neurons,astrocytes can transform into reactive astrocytes,also known as neurotoxic astrocytes or Α1-like astrocytes (Liddelow et al.,2017).C3 serves as a marker of A1-like astrocytes.Although some scholars argue that the simplistic division of astrocytes into A1 and A2 types is not entirely scientifically valid,the transformation of astrocytes into a neurotoxic phenotype has been successfully demonstrated in multiple studies (Guttenplan et al.,2021;Burda et al.,2022).After attaining a neurotoxic phenotype,reactive astrocytes secrete inflammatory cytokines,exacerbating the inflammatory microenvironment within the tissue.This process is intricately linked to the activation of the NFκB pathway (Li et al.,2019;Fei et al.,2022).In this study,mice with SCI displayed a notable rise in the percentage of C3-positive astrocytes and the release of inflammatory cytokines.Treatment with RUX effectively reduced C3 expression in astrocytes,thereby attenuating the neurotoxic phenotype and the secretion of inflammatory cytokines associated with the NFκB pathway.Notably,the role of RUX as a JΑK-STΑT inhibitor should not be overlooked,particularly its effect on microglial cells.In this study,we preliminarily investigated the effect of RUX on the phenotypic transitions of microglia/macrophages.RUX decreased the quantity of M2 microglia/macrophages,suggesting the potential for a reduction in proinflammatory cytokine release from microglia/macrophages.Forin vitroexperiments,IL-1α,TNF-α,and C1q (Α1IM) were used to stimulate Α1-like (neurotoxic) astrocytic responses.TNF-α can suppress EAAT2 expression in astrocytes,especially in an inflammatory environment,leading to impaired glutamate uptake and triggering excitotoxicity (Ye et al.,2013;Martinez-Lozada et al.,2016).In vitroexperiments demonstrated that A1IM reduced EAAT2 expression in astrocytes,whereas RUX restored EAAT2 expression and enhanced glutamate uptake in astrocytes.These results were validated through immunofluorescence staining.RUX exerts potent inhibitory effects on STΑT3 phosphorylation.Additionally,previous studies have suggested that STΑT3 knockout alleviates glutamate-induced excitotoxicity in the dorsal CA1 region and rescues spatial memory deficits in mice with methamphetamine withdrawal (Shi et al.,2021).Therefore,we speculated that STΑT3 is crucial for the effects of RUX in promoting EAAT2 expression.Bothin vitroandinvivoexperiments confirmed the substantial suppression of STΑT3 phosphorylation upon RUX treatment.These findings validate that RUX restores EAAT2 expression in astrocytes and enhances their glutamate-clearing capability by inhibiting STΑT3 phosphorylation.Furthermore,to examine the neuroprotective effects of RUXin vitro,we designed a co-culture model of astrocytes and neurons.The results of calcium imaging,ROS detection,apoptosis-related protein assessment,and neuronal complexity assessment collectively indicated that RUX alleviated glutamate-induced excitotoxicity by restoring EAAT2 expression in astrocytes.
This study has several limitations.This study was conducted using animal and cell models;therefore,further investigation is warranted to facilitate translation of the findings to clinical practice.Moreover,the precise mechanism of the neuroprotective effects of RUX should be comprehensively elucidated.Clinical trials may facilitate the development of optimal treatment strategies for SCI.Additionally,comparative studies,especially those comparing the efficacies of RUX and other potential agents,may help to identify the most effective treatment agent for SCI.
In conclusion,this study reveals that RUX exerts neuroprotective effects and promotes functional recovery following SCI.RUX may exert therapeutic effects by improving the inflammatory microenvironment,restoring EAAT2 expression by inhibiting STΑT3 activation,restoring glutamate homeostasis,and reducing excitotoxicity.
Acknowledgments:We thank the Public Experimental Center of Jiangsu Provincial People’s Hospital for providing the Thunder Imager fast highresolution inverted fluorescence imaging system instrument and other instruments and equipment.
Author contributions:Conceptualization,methodology and writing original draft:JC,TS,JC,XC;methodology and writing original draft:JC,XY,JL;investigation and resources:JF,BW,CW,SZ;visualization and supervision:HC,ZW,YX.All authors read and approved the final version of the manuscript.
Conflicts of interest:The authors declare that they have no conflict of interest.No relevant financial activities outside the submitted,and manuscript is approved by all authors for publication.
Data availability statement:All relevant data are within the paper and its Additional files.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Daniel J.Hellenbrand,University of Wisconsin,USA;Maurice N N Collins,University of Limerick,Ireland;Seth Herr,University of Miami Miller School of Medicine,USA;Sílvia Sousa Chambel,University of Porto Faculty of Medicine,Portugal.
Additional files:
Additional Figure 1:The purity of primary astrocytes and neurons is confirmed to be greater than 95% by immunofluorescence staining.
Additional Figure 2:RUX inhibits the M1 polarisation of microglia/macrophages after SCI.
Additional Figure 3:RUX inhibits GlU-induced apoptosis in primary neurons by restoring glutamate uptake in astrocytes.
Additional file 1:Open peer review reports 1–4.
- 中國神經再生研究(英文版)的其它文章
- A sphingolipid message promotes neuronal health across generations
- Krüppel-like factor 2 (KLF2),a potential target for neuroregeneration
- Defined hydrogels for spinal cord organoids: challenges and potential applications
- Neuronal trafficking as a key to functional recovery in immunemediated neuropathies
- Advancements in personalized stem cell models for aging-related neurodegenerative disorders
- New insights into astrocyte diversity from the lens of transcriptional regulation and their implications for neurodegenerative disease treatments

